GMP-EU COMPLIANT SOLUTIONS FOR THE VIETNAM PHARMACEUTICAL INDUSTRY
The global pharmaceutical industry is working overtime to accommodate demand for COVID-19 vaccines and medicines while ensuring that other drug manufacturing does not suffer. epidemic. Siemens, as one of the top technology providers, has developed a comprehensive solution to improve production efficiency, reduce product time to market, and fulfill industry standards. GMP-EU (Good Manufacturing Practices – European Union) standards, for example, are severe industrial standards.
.png)
THE PHARMACEUTICAL INDUSTRY'S KEY STAGES
1. Development & Research
2.Primary Processing
3. Secondary Processing
.png)
Image: Model 3 main stages in the pharmaceutical industry
SIMATIC PCS 7 GMP-EU STANDARD FOR PHARMACEUTICAL BUSINESS
Control solutions in pharmaceutical manufacturing are required to meet the following requirements:
Automated control and control measures play a critical role in the pharmaceutical business at each level. For each individual necessity in the production chain, these systems are used in a variety of shapes and features. The system can be scaled down to a small scale, such as a laboratory control system or a pilot production system, or scaled up to a large scale, such as a factory control system.
Different features are applied to the control systems for each stage of manufacturing. Process control software is frequently used in primary processing systems. These systems come with programming tools and libraries that are specifically built for configuring process controls. The use of integrated solutions for production lines and data collecting is emphasized in secondary manufacturing systems, which are characterized by several independent production equipment.

Image: Monitoring and control systems in the pharmaceutical industry
Process control systems for active ingredient manufacturing
"Process" as a concept – A process is commonly recognized as a stage in the manufacturing process that converts raw materials in the processing industry in general and the pharmaceutical industry in particular. input for a finished or semi-finished product. In comparison to the input materials, the qualities of these products or semi-finished items have been completely modified. The transformation processes take place under chemical, physical, biological, and other conditions, and they take place over a period of time.
As a result, controlling and monitoring manufacturing processes is critical for pharmaceutical companies to ensure product quality, cost-effectiveness, and efficiency. results in a reasonable amount of time
These objectives guided the development of the SIMATIC PCS 7 system. The features of SIMATIC PCS 7 are reflected in criteria such as: having rich system building tools; meeting all monitoring and control requirements; high flexibility and customization, ensuring all sizes from small to large and meeting all specific production requirements; the system is designed to be open to allow integration and information exchange with other systems; and the system is designed to be open to allow integration and information exchange with other systems.
.png)
Image: SIMATIC PCS 7 is Siemens' flagship "process automation" system
For final manufacturing systems, line integration solutions are available.
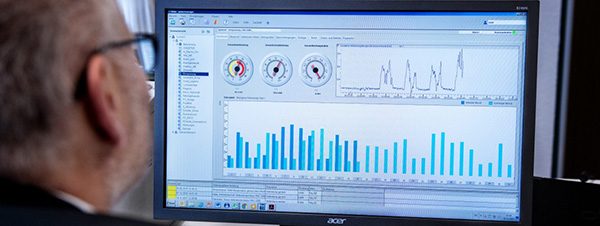
SIMATIC WinCC, a central data gathering and monitoring system, is the key component of the integrated chain solution. WinCC, as one of Siemens' key solutions, connects all machines and production clusters in the production line, allowing for centralized data management in accordance with industry standards. the following criteria:
• Recipe and production parameter management
• Aggregation and analysis features
• Set up batch/batch records
.png)
Image: Display data in the form of charts and graphs for analysis and evaluation
ESTEC is a SIEMENS authorized partner in Vietnam, supplying and integrating digital solutions for the pharmaceutical industry, as well as assisting pharmaceutical enterprises and manufacturers with solutions. GMP-EU standard requirements are met.
We are always ready to support customers who are in need of upgrading production systems, optimizing working processes and factory operations, as well as assisting manufacturers in meeting GMP-EU standards, contributing to the development of the pharmaceutical industry in particular as well as the Vietnamese industry in general, with a team of experienced and well-trained engineers from SIEMENS foreign experts.
If you're serious about the above solutions and have queries, please contact us right away for further information.